Rakeiora is a pathfinder project looking for the best ways to incorporate new genomic-based medicine into New Zealand’s healthcare system. Its vision is to improve wellbeing and reach towards equity in health outcomes by accelerating research and practice of precision medicine in Aotearoa New Zealand.
Rakeiora comprises of two research projects in primary and tertiary health to collect real-time data, and the development of a plan to use health and whakapapa data to test computational systems in order to create a pilot Precision Medicine Research Platform.
E tipu e rea mo ngā rā o tō ao
Ko tō ringa ki ngā rākau ā te Pakeha Hei ara mō tō tinana
Ko tō ngākau ki ngā tāonga a ō tīpuna Māori
Hei tikitiki mō tō māhuna
Ko tō wairua ki tō Atua, Nānā nei ngā mea katoa
Grow up and thrive for the days destined to you.
Your hands to the tools of the Pakeha to provide physical sustenance.
Your heart to the treasures of your Māori ancestors as a diadem for your brow
Your soul to your God, to whom all things belong.
What is meant by precision medicine?
Genomic Medicine (also known as precision medicine or personalised medicine) uses an individual's genetic information to help guide healthcare providers about genetic contributions to a patient's health. This makes healthcare more targeted, based on specific information about each individual’s susceptibility or resilience to disease, or their response to therapeutic interventions.
Why is precision medicine important?
Globally, precision medicine is already helping to diagnose and tailor treatment for inherited diseases and to understand genetic impacts on responses to drugs, all in a much more individualised manner.
A full picture of the individual they treat gives the health professional greater decision making ability - not just from symptoms and test results but also the health history of them and their whānau - what medicines, treatments and lifestyle changes worked in the past, and what ones didn't.
For example:
- Whānau may occasionally have a genetic predisposition to a disease, inherited from their parents, even if they are currently healthy. Precision medicine can allow them to choose non-invasive screening tests to detect the disease early if it does develop, or to choose ways to prevent the disease from developing.
- Some people have genetic differences that make them more susceptible to side effects from medicines. Precision medicine can allow people to be aware of these genetic differences, and allow their doctors to alter how medicines are used to avoid side effects – this is called ‘pharmacogenomics’.
- Today, people with cancer can take highly effective anti-cancer drugs that target the specific mutations in their tumour cells. Precision medicine can identify these mutations to allow the best choice of drug for each patient. This also helps patients avoid expensive drugs with major side effects, which they may not benefit from.
How is precision medicine being used in Aotearoa New Zealand?
Precision medicine is not yet widely available in Aotearoa New Zealand.
There are some genomic medical studies here; this research is often being used to help patients at the same time. But the new diagnostic and screening tests and new therapies from these research trials still need to be comprehensively incorporated into our health system.

As we catch up with overseas trends, it is anticipated that Aotearoa New Zealand’s health services will offer more testing options to prevent, diagnose and treat patients on issues important to our population, including heart disease, diabetes and cancer. Having these appropriately designed tools and processes will specifically help address significant inequities in health care in Aotearoa New Zealand.
What’s needed to make precision medicine available to all New Zealanders
We are on the cusp of a significant change in individualised care in managing health and wellness for people in Aotearoa New Zealand.
However, making precision medicine available to our entire population requires new tools and practices and powerful computing resources.
- It requires systems that bring together our medical records, genetic information, the changes caused by any diseases we have and our family health history - whakapapa.
- It has to consider Aotearoa New Zealand's unique people and culture and build on Tikanga and Te Tiriti frameworks that allow all people to participate in the research comfortably and with confidence in the control, safety and sovereignty of their data. It requires protocols and processes for storing and controlling data that understand and meet Māori aspirations and rights under Te Tiriti o Waitangi.
- And it needs to offer all Aotearoa New Zealand genomic health researchers the same robust processes and protocols for managing a range of people's health information.
This hasn’t been done in Aotearoa New Zealand before.
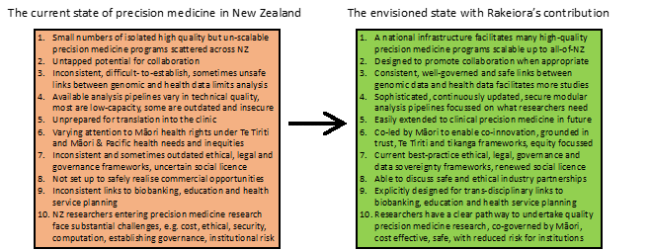
The Rakeiora vision includes 10 specific ways to advance the state of precision medicine in Aotearoa New Zealand
The Rakeiora project
What we’re doing
Rakeiora is a name provided to the project by Selwyn Parata, Chair of Te Runanga o Ngāti Porou and Dr Huti Watson, Research Co-Ordinator Te Rangawairua o Paratene Ngata Research Centre.
The Rakeiora project is incorporating the best overseas genomic health knowledge and systems for our own unique needs to produce a carefully planned comprehensive platform that specifically work within Aotearoa New Zealand's health system and across different health settings.
Our research projects are investigating new ways of testing patients and analysing their DNA, at a very large scale. By then testing and refining this pathway using real data including whakapapa information shared by patients and their whānau, a system is being generated for engagement, consenting, tissue processing and storage, DNA sequence analysis and interpretation, with built-in methods for ongoing improvement.
This approach supports the best possible precision medicine practice right now, while building better precision medicine opportunities for future use.
“This investment is the first step to enabling researchers to translate genomic knowledge into health practices that advance the wellbeing of all New Zealanders, and in particular address the country’s health inequities by developing genomic tools that put the needs and priorities of Māori at its centre.” The Honourable Dr Megan Woods.
What we’ve achieved
1. Co-development: Rakeiora explicitly involves co-development and co-innovation between Māori and non-Māori leaders. This brings mātauranga Māori science and biomedical science together to generate a much better way of using all information available about genes and health, including whole genome sequences, whakapapa and health care information. The more that precision medicine research can be led by, and involve, Māori and Pacific people, the more likely it is that future precision medicine can meet their health rights and needs.
2. Consultation and engagement: The design uses a participatory approach, with widespread consultation amongst Aotearoa New Zealand health professionals and research groups and incorporating global learnings.
- We have been consulting with the biomedical scientific community to define the scientific attributes of the system, gauging the social licence for the establishment of a research platform in precision medicine.
- We are understanding the current environment and have created an international perspective on the evidence for genomically-informed medicine improving health outcomes, with examples of rollouts of precision medicine clinically worldwide. And we have surveyed the level of activity presently in Aotearoa New Zealand, including a gaps analysis.
- We have looked at international best practice for the technical attributes of the system, defined in collaboration with our NeSI partners.
3. Research projects: Two test exemplars in primary and tertiary healthcare have been established to highlight potential gaps in capability and infrastructure. These are generating data that is evidence of the value, and that demonstrates a genomically-informed precision medicine research process.
4. A pilot platform: A first-generation stand-alone scalable computational platform has been set up with co-design, governance, data sovereignty, and co-innovation incorporating mātauranga whakapapa. This Aotearoa New Zealand Precision Medicine Research Platform is drawing together and analysing relevant information on large numbers of people, producing results that are meaningful, useful and precise.
- We have developed some of our own systems alongside best-in-world systems, using a participatory action project design methodology.
- We have been defining the attributes of a Aotearoa New Zealand Precision Medicine Research Platform – Māori, scientific and technical, including established international security standards. We are envisaging and making recommendations around scalability and challenges to grow the prototype in to a National Platform in a culturally appropriate and fully Te Titiri compliant and respectful manner.
- Central to this is genuine involvement by Māori in the design, operation and governance of the platform, the establishment of culturally appropriate consent procedures, policies and structures that enable Māori Governance, and data sovereignty and security attributes that align with Māori expectations and international standards.
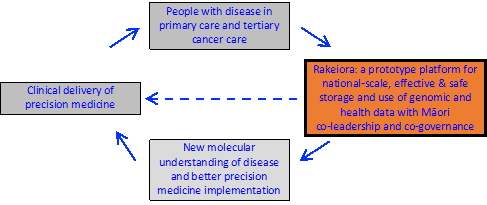
The mission of the Rakeiora genomic data platform, facilitating genomically informed precision medicine research for NZ - enabling a cycle of research, new knowledge generation and clinical care to benefit people with disease.
About the Rakeiora research and infrastructure studies
Primary Care project - partnership with Ngāti Porou Orangaa
We want to work out how to merge whakapapa, genetic and health care information, in a way that is safe, secure, culturally acceptable and implements data sovereignty principles. And we want to turn that process into a system – basically a way that researchers can use to easily combine completely different lots of separate health and genetic information. We want to know how this can be done with cultural integrity, facilitating the maintenance of mana tāngata, mana tīpuna and mana whenua, and with local decision-making for the genomic and whakapapa information.
We need to test it on a small scale to see if that the system works. To do this, we have decided to look at one small part of someone’s genetic code simply to demonstrate the potential of a system – that is not to answer a research question about an individual's genome but rather using the search for one gene as an example of how we go about bringing lots of different information together.
There are many thousands of genes we could look at, but we just want to single out one to test the system - a gene that has an effect on how well a number of drugs (including a drug commonly used to thin the blood in the context of established coronary artery disease), works in people at an individual level.
Working with Ngāti Porou Oranga, we have invited a group of people in Tairawhiti to volunteer to provide their whakapapa information and their hupe (saliva).
- From that sample we get DNA.
- From within their whole genome sequence produced using the DNA we specifically look at a particular gene (CYP2C19) to identify the presence or absence of specific variations.
- We then take that part of the genetic piece of information and look at how we can best combine this with their personal health records securely.
- From that we create a secure place for the data to be stored and accessed.
- Then we work out the best way to report the combined information back to the health professional. Combining such data can give indications on how appropriate the prescription of certain drugs is for individual people.
- We work out how patients access the information and how it should be managed in the future.
- Individuals who volunteer for the project and have a DNA test will get a report about the gene and how it could affect their response to specific drug treatments. The information (genetic factors found within the gene CYP2C19) will be embedded into their medical records, for future reference.
- We also ask participants to share their whakapapa so that we can more accurately predict potential health outcomes.
Tertiary care project – with cancer patients and their doctors in Auckland
Cancer precision medicine, sometimes called precision oncology is already one of the most advanced applications of genomics to precision medicine. But currently we have no way to gain national benefit from the many unconnected programmes in Aotearoa New Zealand that are sequencing cancer genomes using targeted panels of cancer-related genes. We have also not yet scaled up to Whole Genome Sequencing (WGS), and assessed its benefits (WGS is the process of determining the all of the DNA sequences in a genome at once).
The Tertiary Care Project is co-led by Dr Helen Wihongi and Professor Cris Print, with culturally appropriate tikanga and governance frameworks along with detailed standards of practice developed by a rōpū (group of leaders) named Ira Tātai Whakaheke, chaired by project co-leader Dr Helen Wihongi (Director Māori Health Research: Te Whatu Ora Waitemata and Te Toka Tumai Auckland).
Processes and database are being designed to work on a national scale, with analysis of genomic data linked to health care information incorporating primary care project data.
This research is building on the foundation of several currently active Auckland-based projects that sequence DNA; the goal to benefit current and future oncology patients:
- NETwork is a New Zealand-wide alliance of cancer patients, clinicians and scientists, co-led by Drs Ben Lawrence and Cris Print, which combines scientific expertise and clinical practice to improve outcomes for people with neuroendocrine cancers and other solid tumours. The project involves a New Zealand-wide patient data register linked to clinical and genomic sequencing data, including engagement with patient groups and Māori health leaders.
- Molecular Tumour Boards, started by the NETwork project, are clinical meetings where genomic information is analysed in depth then discussed by cancer scientists and clinicians, to help patients and their doctors make better clinical decisions on the use of modern molecularly-targeted therapies, immunotherapies, or entry into genomic-directed clinical trials.
- PROSPER and MoST are genomic-directed clinical trials with clinical lead Dr Michelle Wilson. They implement genomic precision oncology in Auckland across tumour types. DNA information is collected from up to 100 patients over a two-year period, after optimising pipelines and protocols. This will produce a broad coverage of different cancer types, allowing genome sequence data to be used for research and practice in different ways to test scalable data storage and analysis.
- Te Ira Kāwai is the Auckland Regional Biobank, with tissue and data protocols developed over several years under Māori co-leadership. It manages our consenting and data collection, using the tissue collection and clinical data processing protocols developed by Rakeiora.
Patients in the tertiary care project will be provided with clinical-grade tumour sequencing through their doctors, using a best-in-world 500-gene cancer deep sequencing test as well as a clinical-grade sequencing test of genes which examine how cancer drugs work in them as individuals. This research will consider whether cancer patients benefit more from targeted DNA sequencing panels of a few hundred genes well known in cancer, or from Whole Genome Sequencing.
The project is establishing consent processes that can involve whānau/families for safe and acceptable posthumous kaitiakitanga (governance) and use in research of consented DNA sequence and health data if patients choose - sadly, many of the patients who consent to analysis of their DNA and clinical information will pass away during the lifespan of a research programme.
Rakeiora computing infrastructure
Rakeiora requires a national-scale computing infrastructure. This is being developed by Aotearoa New Zealand’s National eScience Infrastructure (NeSI,) in partnership with the Rakeiora team and mana whenua.
The project has where possible re-used national-scale overseas initiatives (such as the GEN3 data commons, ‘Keycloak’, REMS and the GA4GH international data standards and htsget API). NeSI has also generated parts of this national-scale computing infrastructure, developed to align with the indigenous data sovereignty attributes we have now identified.
After extensive consultation, we have chosen a ‘walled garden’ approach to this infrastructure. This means that all genomic data and health data, and its analysis, are undertaken only within this computational environment – the data never leave and are certainly never downloaded to a researcher’s own computer or a hospital clinical laboratory computer.
The GA4GH authentication methods adopted by Rakeiora involve rigorous permission systems for both investigators and projects that use Rakeiora data. This allows secure access to federated data stored on different servers as well as precise tracking of what data is accessed, what analyses are performed and appropriate permissioning before results can be exported.
This sophistication and scale is producing a system which is effective for precision medicine research, and adaptable to a wide range of different research problems and precision medicine uses, which makes it attractive to researchers and clinicians.
Its modular nature will allow linkage (with consent) to other health data systems currently under development in Aotearoa New Zealand. This approach in particular addresses transparency of use and control over narratives. Lack of these features in other precision medicine platforms worldwide has led to significant problems for patients and research participants, including indigenous communities; learning from these negative experiences is key to avoid repeating them.
About the Rakeiora name
Rakeiora is a descendant of Toi, and was a renowned Tohunga and navigator. Through his leadership he was able to display his uncanny skilled knowledge of his surroundings, as a Tipuna Rakeiora depicts his wellness through his journey as a pathfinder. A visionary, he led his people to new horizons to discover new lands, places that would sustain life where they could stand and flourish. The Waka Hourua carried Rakeiora to the fulfilment of his visions and aspirations.
This talks to navigation, steering the waka into uncharted territory. Ngāti Porou and other iwi, along with partners from University of Otago, University of Auckland, Te Whatu Ora Waitemata and Te Toka Tumai Auckland, ESR, Genomics Aotearoa and MBIE want to chart a sure course in a safe way that protects our treasures for the benefit of all.
“Aeons ago our ancestors raised their vision beyond the horizon and set their sails to discover new land, a place that would sustain life, where they could stand and flourish. Genomics is the new horizon and within it lies new ground waiting to be explored; yielding treasure that will enrich our lives. Our tipuna closely held the secrets of navigating a pathway across the ocean so they could find their way back again. As it was then, so it is today. Ngāti Porou, with our partners, want to chart a sure course, a safe way, and a secure place to store sacred treasure and utilise it for wide benefit.” Teepa Wawatai, former Ngāti Porou Hauora Chair.
Whakapapa
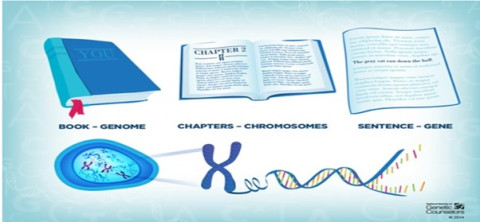
Our genes contribute to who we are and tell us about our connections to each other, and so our DNA sequences have so much to tell us.
Genes: Genes are the instruction codes for our body. Think of genes as the many sentences that make up a book - one with lots of chapters. Much like whakapapa, these genes are inherited from our parents and our ancestors. Each gene carries a set of instructions for building individual characteristics, like eye and hair colour, and for conditions we can inherit.
DNA: Genes are made up of a substance called DNA. Everything we need to know is in the letters than make up that DNA – like the words which make up those sentences for the book.
Genome: The full set of genetic instruction is called a genome – the entire book. Humans have around 20,000 genes in our genome. As with whakapapa, these genetic codes hold your family history – they are passed on to you through tipuna/ancestors to your parents and on to your tamariki and mokopuna.
Genetic variation: Inheritance means that parental genes are mixed in many different ways between family members. And that means relatives will have small but significant differences in the genetic codes. These few small differences, known as genetic variation, are enough to make each person unique. It’s these unique combinations of variation in our genetic codes that points us in the direction of why some humans are more likely to have a particular disease than others. DNA inherited from our tīpuna/ancestors merges and divides into different combinations, just like a braided river that comes down from the mountain. Those combinations of whānau inheritance flow on in different forms onto our mokopuna. Also passed on from our tīpuna are genes that improve or reduce our resilience to modern health conditions. Because everyone inherits a unique set of such genes, there is variation within and between whānau in resilience. This is also why we don’t all look exactly the same, and also why our resilience to heritable condition varies.
Whānau dynamics: Much like whakapapa, genetics is inherited through generations from our tipuna/ancestors. Unlike whakapapa, genes are mixed differently between individuals from the parents. This means that an individual can have a very different genetic makeup from their sibling. This is very similar to ira tāngata, the essence of the individual. Despite these vital individual characteristics, whakapapa remains constant, much like ira atua and ira tīpuna, while individual DNA has very dynamic elements. So, we are increasingly using the term ‘Te Mata Ira’ (the faces of our essence) to describe the richness of genomes, in Māori terms.
Personalised medicine: Whakapapa is very important to deliver personalised medicine (PM) for Māori. Together with whakapapa, DNA will allow us to more accurately predict the resilience of nga uri whakatipu me nga uri ake nei (future descendants). Personalised medicine = whakapapa + DNA. Potentially, this information can inform whānau about conditions before they arise, so that interventions can be undertaken to improve resilience. An existing example is the stomach cancer gene in various Māori whānau in Tairawhiti and Bay of Plenty, where those carrying the gene are offered surgery to remove the risk of stomach cancer. Interventions are specific to various conditions.
Cultural benefit to understanding DNA alongside whakapapa: It is important that, much like our whakapapa records, new generations will participate in the care of our biological whakapapa. A key element of maintaining mana kaitiaki over the data is by creating connection with its origin. Its human, tāngata whenua origin grounds the sample and data within its cultural context. By maintaining its point of origin beside the genomic data, we retain and maintain its mana. We also create the pathway for ongoing whakawhānaungatanga (creating and maintaining positive relationships) between the genomic data, the originator and researchers and their institutions. This is the primary practice for long term mana kaitiaki being maintained. This study is also considering what can be done to ensure inter-generational mana kaitiaki.
Mana enhancing research: A component of the Rakeiora study examines the cultural and technical appropriateness of including mātauranga whakapapa in precision medicine. Overseas research has shown that including information from relatives improves prediction of health outcomes. Māori have very deep knowledge of whakapapa. However, whakapapa is a culturally defining concept and is therefore tapu (restricted) thus it is necessary to define appropriate mechanisms (tikanga) to ensure it is appropriately used and therefore outcomes from precision medicine that incorporates whakapapa are mana enhancing.